5. Comparison of interventions in certain groups of patients
5.1. Congenital middle ear and outer ear anomalies
5.1.1. Surgical repair or amplification?
When counselling patients with hearing loss caused by congenital ear anomalies (like aural atresia), firstly, reconstructive surgery should be considered. Congenital ear anomalies might vary from mild (middle ear anomalies) to severe (atresia of the ear canal) with an associated air-bone gap from 40 to 65 dB. Amongst otologists, reconstructive surgery of congenital anomalies is considered as very challenging because of complicated anatomy and risk of complications. In audiometric terms, benefit is often limited and not stable over time. Theoretically, surgery is successful if the air-bone gap is closed, but mostly, post intervention, an obvious air-bone gap remains. To define a successful outcome after atresia repair, a rather broad criterion is used: if the remaining air-bone gap is 30 dB or less, the outcome is considered as sufficient (Nadaraja et al., 2013). In their systematic review (including 35 studies), Nadaraja et al. showed that this criterion is fulfilled in approx. 50 % of the operated patients, when evaluated 12 months after surgery. In 1999, based on a review of the literature and expert opinions, Declau et al. (1999) already concluded that atresia repair was only safe in experienced hands and they suggested that alternative options like percutaneous BCDs should be considered more often. That conclusion has been underlined by Evans & Kazahaya (2007). They reported that the majority (93%) of their operated atresia patients still needed some type of hearing device. Further evidence came from the Nadaraja et al. study. In their systematic review, they compared studies with Baha application and those using atresia repair, and concluded that the results with Baha were significantly better; the mean improvement (over studies) in hearing thresholds was 38 dB for Baha application and only 24 dB for atresia repair.
Apart from aural atresia, minor congenital (middle ear) anomalies might occur; e.g. isolated congenital stapes ankylosis or mobile stapes with ossicular chain anomalies. Thomeer et al. (2010; 2011; 2012) published one-year post-intervention audiometric data; the mean remaining air-bone gap was 15 to 20 dB. One year after surgery, 65 to 75% of the operated ears had a PTA (air conduction) of 30 dB HL or better. However, as stated by Thomeer, hearing thresholds might have been better with hearing devices.
In case hearing aid fitting is considered in minor congenital middle ear anomalies, the first amplification option is a conventional BTE. If the air-bone gap exceeds 35 dB HL, a percutaneous BCD is the better option; the larger the air-bone gap, the higher the profit of a percutaneous Baha/Ponto device over a BTE (see paragraph 2.2).
Ten years ago, as an alternative amplification option, Colletti et al. (2006) introduced the VSB with its actuator (FMT) directly coupled to the round window of the cochlea (see also paragraph 2.3). Amongst others, Frenzel et al. (2009) and Colletti et al. (2010) reported their experience with VSB in aural atresia cases and reported that this amplification option was safe and effective.
Concerning this VSB application, several coupling options for the FMT to the cochlea have been introduced, either connected to the mobile stapes (footplate) or to the round window. If there is a choice, there is a preference for a coupling to the oval window because of higher output (e.g. Hüttenbrink et al., 2008). A long-term evaluation showed that all FMT couplings were stable, with the best effective gain for incus coupling (Bush et al., 2017).
Transcutaneous BCDs have also been used, but so far little has been published (Dimitriades et al., 2016). A proper comparison with the percutaneous BCD is lacking.
It should be noted that to obtain a good hearing results, the prescription model introduced in paragraph 4.3 might be taken as a starting point.
5.1.2 MRI compatibility and stability of these implantable devices
An important issue to consider when choosing an amplification option is MRI safety. MRI is a powerful and increasingly popular diagnostic tool. Statistics show that the incidence of MRI scanning increases steadily over the years. In 2010, the incidence was 51 MRI scans per 1000 subjects (for the Netherlands, CBS, 2012).
Doshi et al. (2014) reviewed literature and manufacturer’s information on MRI compatibility of percutaneous and transcutaneous BCDs and middle ear implants. The percutaneous (titanium) implant is MRI safe; in contrast, the middle ear implants are not MRI safe. Early 2015, a new VSB implant was introduced, the VORP 503, which is MRI safe up to 1.5T. The transcutaneous bone-conduction implants are MRI compatible either up to 1.5T (Bonebridge, Baha Attract) or 3T (Sophono implant).
Whether or not a MRI scan harms the implant or actuator is of importance but also the degree of distortion of the MRI scan by the implanted device. The percutaneous implants affect the MRI image only locally (radius of 15 mm) while the transcutaneous implants, comprising powerful magnets, distort the MRI scans more significantly, with a radius of 10 to 15 cm (Doshi et al., 2014).
Concerning stability of the implanted parts; long-term data for most systems are relatively scarce. Table 5.1 shows some data calculated from literature. Per listed study, the table presents the summed number of reported revision surgeries (for whatever reason) plus the reported number of lost implants that were not replaced (revisions +). Next, that number is divided by the summed years of follow-up of all the patients in that study. That number, the revision ratio, is presented in the Table. Concerning the percutaneous Baha device, Wazen et al. (2008) published data of a North-American multicenter study. In 2014, Nelissen et al. reported the stability outcomes of a European multicenter study, using the new implant Baha BI300 implant. Hultcrantz & Lanis (2014) published their first results on long term stability of percutaneous implants, placed without tissue reduction around the implant. More recently, Strijbos et al. (2020) published an overview with the Oticon percutaneous coupling. In half of them the traditional approach however without tissue reduction was used while in the others an experimental approach was tried out.
The Table shows that with the traditional approach with skin reduction, revision surgery is relative rare; just one revision in 43 years of follow-up, or even better, while the more recent approaches without reduction of the skin show somewhat poorer results. The numbers are too low to draw any firm conclusion. Table 5.1 presents the results as obtained in adults. From the data of Dun et al. (2012) it is calculated, that the revision ratio in children using Baha is almost 3 times higher than that in adults.
Table 5.1. Implant stability data obtained in adults: number of revisions divided by the total follow-up
| Device, # of implants | Revisions | Total follow-up | Revision ratio | Reference |
| Baha (n=218) | 23 | 990 yrs | 1 in 43 yrs | Wazen et al., 2008 |
| Baha BI300 (n=77) | 3 | 230 yrs | 1 in 77 yrs | Nelissen et al., 2014 |
| Baha, without skin reduction (n=12) | 2 | 60 yrs | 1 in 30 yrs | Hultcrantz & Lanis, 2014 |
| Oticon, without skin reduction (n=30) | 1 | 55 yrs | 1 in 55 yrs | Strijbos et al., 2020 |
Concerning middle ear implants, recently, the number of (mostly medical) problems have been reported by e.g. Bernardeshi et al. (2011), Colletti et al. (2013), Mlynski (2014), Zwartenkot et al. (2014) and Brkic et al. (2019). The derived revision ratios were between 1 in 15 years up to 1 in 28 years of follow-up. These figures are obviously worse than those reported for the percutaneous BCDs, especially whenever the approach with skin reduction is applied see Table. Growing pains might play a role, especially as there are still several different surgical approaches.
Concerning the other devices (Bonebridge, Sophono), systematically obtained stability data are lacking (Summer 2016).
In case of congenital conductive hearing loss, including aural atresia, often, surgical repair will lead to inferior hearing. So far, only the stability of percutaneous BCDs is well documented, at a level that is an obvious challenge for the other amplification systems. Concerning MRI compatibility, percutaneous BCDs are the best option, not only because titanium implants are MRI safe but also because they affect the MRI scans only marginally.
5.2 Chronic otitis media with effusion and suppurative otitis media
In most cases, otitis media with effusion (OME) is a transient disease, affecting mainly children. Most children recover spontaneously or they are surgically treated (grommet insertion). However, in some patients, OME might be (semi) chronic, e.g. in children with Down syndrome and children with cleft palate (Sheenan & Hans, 2006). Then provision of a (temporary) hearing device might be beneficial. The OME-associated (conductive) hearing loss in children typically varies between 15 dB HL and 35 dB HL what suggests that BTEs can be fitted. A BCD on a headband or softband has also been advocated (Ramakrishnan et al., 2006). The advantage of a BCD is that when the hearing loss fluctuates over time (not unlikely for OME), BTEs have to be refitted, in contrast to BCDs. Note, however, that aided thresholds with conventional transcutaneous BCDs typically lay around 25-30 dB HL, thus close to the unaided thresholds (Snik et al., 2008). Indeed, according to the data of Ramakrishnan et al. (2006), who provided children with OME with BCDs on headbands, it was not a great success.
In patients with chronic OME, BTEs are a good amplification option
Chronic suppurative otitis media (CSOM) is a long-standing middle ear inflammation that results in periods of discharge from the ear. The tympanic membrane is perforated. Chronic suppuration can occur with or without cholesteatoma. Treatment of such patients is mainly directed at the inflammation and, eventually, cholesteatoma removal. This disease might lead to substantial conductive or mixed hearing loss. In patients with suppurative otitis media, BTEs with occluding ear moulds are contraindicated because an occluded ear canal might have an adverse effect on ear discharge. Chronic ear discharge may lead to cochlear damage (e.g. Papp et al., 2003).
While BTEs are not a safe choice, percutaneous BCDs can be used and the outcomes are favourable (Snik et al., 2005). Literature has shown that replacing BTEs by percutaneous BCDs had a significant positive effect on the ear discharge (Macnamara et al. 1996; Mylanus et al. 1998; McDermott et al. 2002). The observed reduction of infections resulted in significantly less outpatient visits (Hol et al., 2004), what is an important advantage in terms of cost-benefit as well as in patient’s convenience.
Another amplification option is middle ear implants, like the VSB device, with the actuator coupled to one of the cochlear windows. This device has successfully been implanted after subtotal petrosectomy with obliteration of the ear cavity with abdominal fat (e.g. Linder et al. 2009 and Ihler et al., 2013). Minor technical and medical problems were reported. Ihler et al. reported one revision surgery in their 10 implanted patients. Mean gain at threshold level (or the effective gain; for its definition, see Appendix 2) was comparable to that of the VSB applications in other types of mixed hearing loss (+2 dB and +4 dB). With the Sophono device (transcutaneous BCD) applied in 10 patients after subtotal petrosectomy, a mean effective gain of -13 dB was found (mean PTAbc was 29 dB HL; Magliulo et al., 2015), suggesting a less powerful application.
Concerning MRI compatibility, percutaneous BCDs are the best option (see paragraph 5.1.2), especially for ears that need follow-up with MRI scans (e.g. after cholesteatoma surgery).
To obtain a good hearing result, it is suggested to use the prescription model introduced in paragraph 4.3, at least as a starting point.
For patients with chronic running ears (CSOM) who need amplification, percutaneous BCDs are the first treatment option. Middle ear implants can be used as well, although implant surgery is complicated by the fact that a stable, infection-free middle ear has to be established, first of all.
5.3 Otosclerosis
5.3.1. Introduction
Otosclerosis is a disease that leads to mixed hearing loss. Large variations in hearing loss have been reported and hearing loss might deteriorate over time at different rates (e.g. Rotteveel et al., 2004; Sziklai et al., 2009). The conductive part is caused by stapes fixation. Although some twenty-year-old patients with otosclerosis might already have a SNHLc of 30 dB HL (Iliadou et al., 2006; Topsakal et al., 2006), approximately 90% of the patients suffer from a predominantly conductive hearing loss and a presbyacusis-like deterioration of SNHLc with relatively early onset (Kursten et al., 1994; Aarnisalo et al., 2003; Topsakal et al., 2006). The other 10% suffer from an obvious mixed hearing loss; around 1.5% of these patients develop a profound SNHLc, exceeding 70 dB HL, referred to as ‘advanced otosclerosis’.
The generally accepted first treatment option is to place surgically a stapes prosthesis to restore the ossicular chain (stapedotomy). This is considered as a safe and effective intervention that reduces the air-bone gap in most cases to below 10 dB. At the high frequencies, the effectiveness might be somewhat worse (e.g. Strömbäck et al., 2012).
5.3.2. Amplification options
If, after stapedotomy, amplification is still needed, then the first choice is a BTE. In case of advanced otosclerosis, a powerful BTE is needed (e.g. van Loon et al., 2014; Wardenga et al. 2020). VBS application with the FMT applied in the classical way (connected to the incus, moving in parallel to the stapes prosthesis) is also an option, as has been shown by Dumon et al. (2009) and Venail et al. (2007).
Kontorinis et al. (2011) and Beltrame et al. (2009) also applied the VBS in patients with otosclerosis, however, with its actuator (FMT) coupled to the round window.
Another option is the use of BCDs. In that case, stapes surgery is not a prerequisite. Burell et al. (1996) used the percutaneous BCD and called it: ‘the third option for otosclerosis’. Since then, little has been published on this application.
Another option was to use the Codacs device; this device, specially developed for patients with advanced otosclerosis is taken of the market 2020. However, still included in this analysis as it was the most powerful treatment option ever.
A literature search was performed aimed at published audiometric outcomes, obtained with any of these amplification options (BTE, VSB, BCD or Codacs; summer 2015); little has been published, especially on BTE fittings after stapedotomy. Figure 5.1 gives an overview of the collected data. Concerning BTE, the data were retrieved from several studies in which stapedotomy was not necessarily performed or unsuccessful (Burell et al., 1996; Lenarz et al., 2013; Busch et al., 2013).
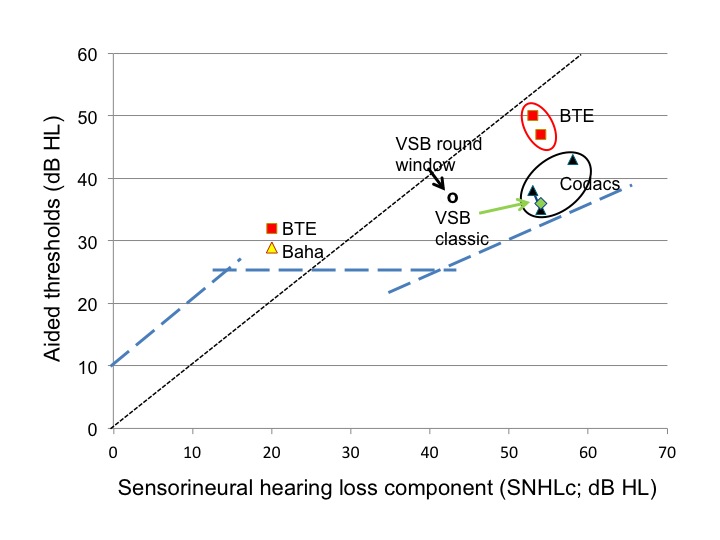
Figure 5.1. The mean aided thresholds presented versus the mean SNHLc of study groups of patients with otosclerosis using: BTEs (red symbols, 3 studies, n=27), Baha HC300 (yellow symbol, 1 study, n=7), VSB with the FMT coupled to the round window (open dots, 2 studies, summed n=7), VSB applied in the classical way (green symbol, 2 studies, summed n=7) and, finally, Codacs device (black round, 3 studies, n=44). Standard deviations (not indicated) varied between 4 and 6 dB in either direction. Target line for aided thresholds is presented as introduced in paragraph 4.3.
Figure 5.1 suggests that application of either the Codacs device (data from Lenarz et al., 2013; Lenarz et al., 2014; Busch et al., 2013) or the VSB with FMT coupled in the classical way (data from Venail et al., 2007; Dumon et al., 2009), are the most effective options because they are closest to the target line. The studies with the FMT coupled to the round window (data from Kontorinis et al., 2011; Beltrame et al., 2009) seem to be somewhat less effective. This suggests that FMT applied in the classical way is a better option, what is in accordance with the experimental findings of Hüttenbrink et al. (2008).
For patients with advanced otosclerosis there is still a debate on what the best option is; stapedotomy plus powerful BTE or cochlear implantation. Van Loon et al. (2014), reviewing the literature and their own data showed that stapedotomy with BTE fitting in patients referred for CI should still be considered; performing stapedotomy and BTE fitting in several of their CI candidates resulted in adequate speech perception. Another recent observation was that patients suffering from advanced otosclerosis and fitted with the Codacs device had speech in quiet scores equal to or better than those of CI users matched with respect to their SNHLc (in the range of 55-75 dB HL). Moreover, speech recognition in noise was obviously better in the Codacs group (Klundt et al., 2015). This suggests that acoustic stimulation might outperform electric stimulation in patients with severe SNHLc.
First treatment option for patients with hearing loss owing to otosclerosis is stapedotomy plus, eventually, the fitting of BTEs. Often, patients with otosclerosis, referred for cochlear implantation, might still benefit from a stapedotomy and the fitting of powerful BTEs. In cases with advanced otosclerosis, speech perception scores with acoustic devices might be better than those obtained with a cochlear implant.
MRI compatibility of the studied devices has been described in paragraph 5.1.3.
Stability. Stability figures have been presented in paragraph 5.1.3, concerning percutaneous BCDs and VSB. Those stability data were not specified according to aetiology. There is no reason to believe that aetiology plays a significant role when looking at BCDs. Concerning the VSB with its transducer coupled to the incus, it is expected that stability factors will not be worse than those published so far for application of the VSB in sensorineural hearing loss. However, Venail et al. (2007) reported a severe deterioration in hearing in one of their five patients who received a middle ear implant after stapedotomy and the patient became a non-user. Stapedotomy in itself is a rather safe procedure; however, severe hearing deterioration might occur in a few per cent of operated ears (e.g. Rotteveel et al., 2004; Kursten et al., 1994).